Technology
Botanical Drug: Source of new products for the pharmaceutical industry
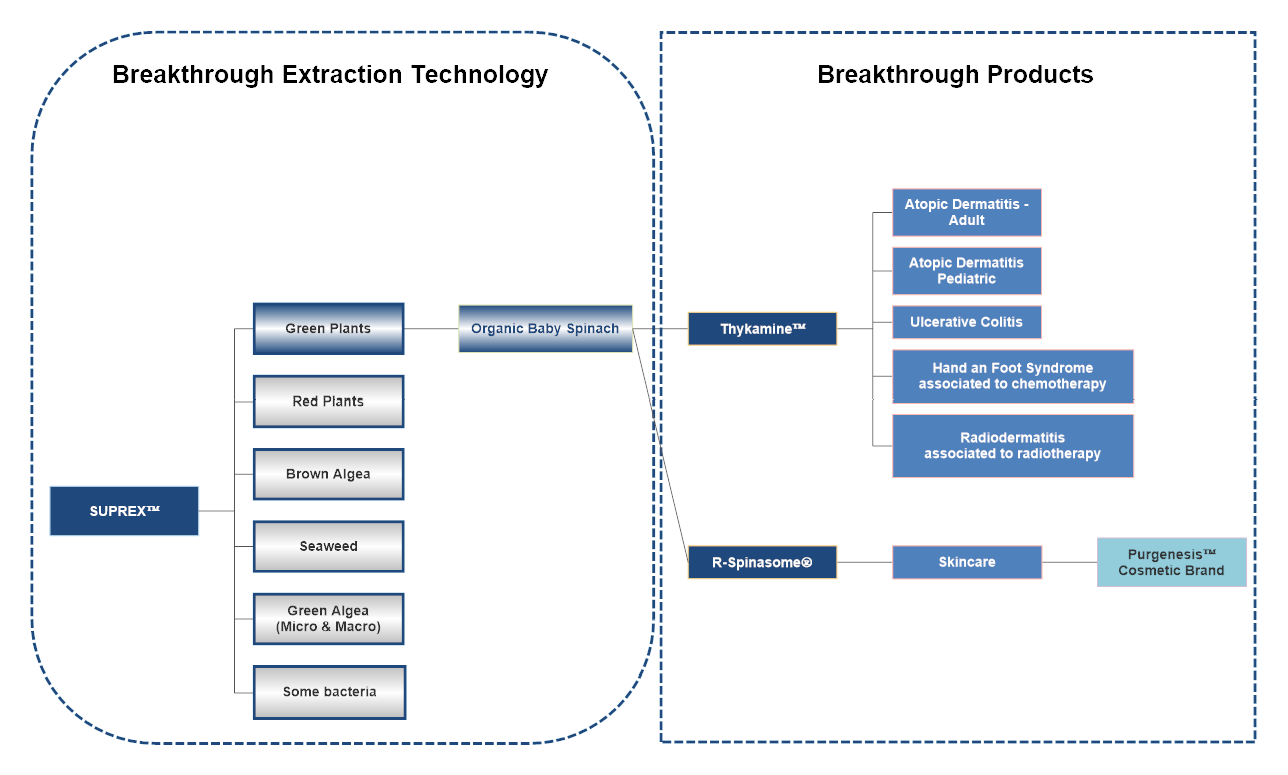
Devonian’s technology is based on a broad-based platform originating from over fifteen years of research. This platform provides a unique process of water-based extraction, purification, stabilization and conditioning of molecular complexes, as Active Botanical Ingredients (ABIs), from plants and algae: The Supra Molecular Complex Extraction and Stabilization Technology (“SUPREX™”). The SUPREX™ technology is protected by a broad IP estate. Each ABI has the potential to target several diseases making it a strong therapeutic platform. Thykamine™ is the first ABI issued from this platform. The potent anti-inflammatory, anti-oxidative and immunomodulatory activities of Thykamine™ have been demonstrated in several pre-clinical studies (in vitro and in vivo pharmacology studies; safety pharmacology; and toxicology studies) as well as in a Phase 1 clinical study in healthy adult volunteers, and in a Phase 2a “proof of concept” clinical study in patients with active mild-to-moderate distal ulcerative colitis.
Devonian has also completed a phase 2 clinical trial in mild-to-moderate atopic dermatitis using Thykamine™ in a cream formulation. Thykamine™ monotherapy achieved positive results by reaching the study’s primary endpoint with statistical significance, delivering significant improvement in skin clearance as determined by the Investigator Global Assessment (IGA). The Trial demonstrated statistically significant results on secondary endpoints of Body Surface Area (BSA), pruritus, POEM and Quality of Life. Thykamine™ was well well-tolerated, safety profile consistent with prior studies. Efficacy and safety profile support advancement into Phase 3 in adult population and the initiation the program in the pediatric patients with Mild-to-Moderate Atopic Dermatitis.
To date, all data support the potential use of Thykamine™ as a therapeutic platform targeting several inflammatory diseases.
While the development of prescription botanical drugs is its core business, Devonian is also involved in the development of high value derma-cosmeceutical products as part of a secondary strategy to generate short-term revenues and optimize manufacturing efficiency.
Devonian owns a state-of-the-art extraction facility with full traceability.